Design Calculation (Sizing) Of A Crystallizer
Problem Statement And Given Data:
Given
the following data and information, it is required to design a forced
circulation crystallizer of the type shown in Figure 1 operating under vacuum.
 |
| Figure 1 Forced circulation (FC) evaporation crystallizer. |
Feed
(an aqueous solution) rate, Qi = 15 m3/h, feed concentration, Ci =
200 kg/m3 solution; feed temperature = 55oC; average
density of the solution = 1100 kg/m3 and average specific heat =
0.90 kcal/kg.oC; operating pressure = 100 mm Hg (660 mm Hg vacuum);
boiling point elevation of the saturated solution = 13oC;
saturation concentration at the crystallization temperature = 250 kg/m3;
magma density allowed, MT = 350 kg crystal/m3 solution;
crystal growth rate determined experimentally under the conditions of the
crystallizer, G = 4.67 x 10-8 m/s; crystal density = 1700 kg/m3;
desired dominant crystal size LD = 0.8 mm; heat of vaporization of
water at the temperature of the crystallizer = 570 kcal/kg; superheat allowed
in the heat exchanger = 3oC; overall heat transfer coefficient in
the heat exchanger U = 1000 kcal/m2.h.oC; low pressure
steam is available for the heat exchanger at 3 kg/cm2 gauge, latent
heat of condensation = 510 kcal/kg.
Solution:
Material balance (basis 1 hour operation)
Solution in = 15 m3 ; solute in = (15 m3)(200 kg/m3) =
3000 kg
Water
in = (15)(1100 - 200) = 13500 kg
Magma
density = 350 kg per cubic meter solution
= 350 kg crystal per
250 kg solute in the solution.
Crystals
produced = [(350)/(350 + 250)] x 3000 kg = 1750 kg.
Solute leaving with mother liquor = 3000 kg - 1750 kg = 1250 kg.
Volume
of solution (clear mother liquor) leaving = 1250 kg / (250 kg/m3,
solubility) = 5 m3
Mass
of solvent (water) leaving = (5 m3)(1100 - 250) kg/m3 =
4250 kg
Mass
of water evaporated = water in with feed – water out with mother liquor
= 13500 – 4250
= 9250 kg
Volume
of slurry leaving per hour = volume of solution + volume of crystals leaving
= 5 m3 + [1750 kg/ (1700 kg/m3)] = 6.03 m3
per hour
Crystallizer Volume
Dominant
size of the product, LD = 0.8 mm = 3GTH ; G = 4.67 x 10-8
m/s (given).
Required
holding time, TH = LD/3G = (8 x 10-4
m)/(3)(4.67 x 10-8 m/s) = 5710 s = 1.6 h
Volume
of suspension in the crystallizer at any time = (6.03 m3/h)(1.6 h) =
9.65 m3
This
is the “working volume” of the crystallizer (note that the suspension holdup in
the pipe line and in the heat exchanger tubes has been neglected). Add 60% to
account for vapour bubbles and froth.
Effective suspension volume in the
crystallizer = (9.65 m3)(1.6) = 15.44 m3
Select
a 3 m diameter vessel (this is to be
checked and changed if necessary) with a conical bottom. Take a cone angle of
45 degrees for the conical bottom.
Volume
of the conical part (radius = depth = 1.5 m) of the tank = (π/3)(1.5)2(1.5)
= 3.53 m3
Volume
of the cylindrical part = 15.44 – 3.53 = 11.91 m3 ; height =
11.91/(π)(1.5)2 = 1.68 m
Add
1.25 m space above the boiling liquid for disengagement of the entrained
droplets.
Total
length of the cylindrical part of the tank = 1.68 m + 1.25 m = 2.93 m say 3 m
Now
check the assumed diameter of the tank.
Absolute
pressure in the vapour space = 760 – 660 = 100 mm Hg = 0.1316 atm
B.P
of water = 52oC. B.P elevation = 130C = B.P of the
solution = 52 + 13 = 65oC = 338 k.
Density
of vapour (steam) at this temperature and pressure, ρv = (18)(0.1316)/(0.0821)(338)
= 0.0854 kg/m3
Volumetric
rate of vapour generation = (9250 kg/h)/(0.0854 kg/m3) =
A
Souders-Brown type equation (Equation 1) is used to determine the allowable velocity
of the vapour without risking entrainment.
 |
| Equation 1 |
For
evaporation under vacuum with a demister, a conservative value of Cv
= 0.04 m/s is used.
Allowable
velocity, uv = 0.04 [(1100
/ 0.0854)0.5 ] = 4.54 m/s
Area
required for evaporation = (1.083 x 105 m3/h)/(3600)(4.54 m/s) = 6.63
m2
Cross-section
of the tank = 7.07 m2. Hence a tank of 3 m diameter is suitable.
Energy balance and heat exchanger area
The
feed liquor enters at 55oC; feed rate = 15 m3/h =
(15)/(1100 kg/m3) = 1.65 x 104 kg/h
Take
the B.P of the liquor, 65oC, as the reference temperature.
Required
heat input to raise the liquor temperature to 65oC,
= (1.65 x 104)(0.9)(65
- 55) = 148,500 kcal
Heat
required for evaporation of water = (9250 kg/h)(510 kcal/kg) = 5.272 x 106
kcal/h
(Superheat
of the vapour produced is neglected)
Heat
of crystallization = (1750 kg/h)(30 kcal/kg) = 5.25 x 104 kcal/h
(absorption)
Total
heat input required, Qh = 1.485 x 105 + 5.272 x 106 + 5.25
x 104 = 5.473 x 106 kcal/h
Heating
steam supplied at 3 kg/cm2 gauge (saturated) = steam temperature =
143 oC
Latent
heat = 510 kcal/kg = steam rate = (5.473 x 106 kcal/h)/(510
kcal/kg) = 10,730 kg/h
Heat exchanger area
Temperature
driving force, ∆T1 = 143 – 65 = 78oC; ∆T2 = 143 – 68 = 75oC;
(∆T)m = 76.5oC
Area
of the heat exchanger = Qh/U.(∆T)m = (5.473 x 106)/(1000)(76.5)
= 71.5 m2
An
outline design of the crystallizer is given in Fig 2 below.
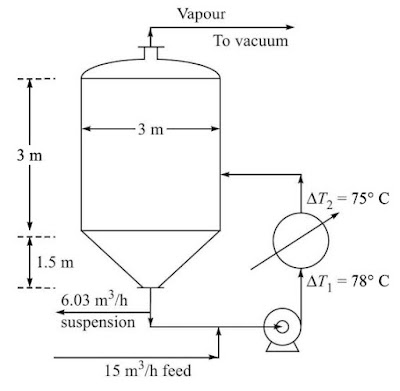 |
| Figure 2 An outline diagram of the crystallizer. |
Read crystallizer selection and design considerations articles for better understanding.


I was trying to understand this concept for so long but was unable to understand it. Thanks for sharing this blog it cleared my concepts.
ReplyDeleteCrystallizers supplier in USA
Alaquainc, which specializes in designing and manufacturing a broad range of different crystallizer systems designs to fulfill diverse client requirements.
ReplyDeleteThe process of crystallization is divided into two stages that are primary nucleation and secondary nucleation where primary nucleation helps in the growth of new crystals and secondary nucleation is the main stage of crystal growth that ultimately leads to the crystals’ mass production.
ReplyDeletecrystallizer
crystallizer supplier in usa
crystallizers made in usa
crystallizers supplier
crystallizers supplier in usa
Thanks for sharing. Really helps lot.
ReplyDeleteevaporator system
An evaporator is a processing machine/device used to convert liquid chemicals such as water into vapors or gaseous forms. The evaporator is a chemical processing machine used by various industries for different types of chemical processing.
ReplyDeleterising film evaporators
falling film evaporators
industrial distillation equipment
distillation supplier
This comment has been removed by the author.
ReplyDeleteThank you for the helpful blog, "Design Calculation (Sizing) Of A Crystallizer." I want you to know that your information is invaluable for aspiring candidates. Keep sharing valuable updates!
ReplyDeleteChandu Biology Classes
I always learn something valuable from your updates. Your perspective is truly refreshing. Groundnut Shell Grinder
ReplyDelete