Crystallizer Selection And Design
Guidelines For Crystallizer Selection:
This is part one of a two-part feature that examines the fundamentals and discusses crystallization equipment selection and design. Part one focuses on the basics, providing guidance for crystallization equipment selection. Part two will focus on the crystallizer design procedure.
The guidelines for crystallizer selection and operation in various chemical industry processes are discussed below.
- Information Required For Evaluation:
Before a potential crystallizer application can be properly evaluated, it is necessary to have certain basic information regarding the material to be crystallized and its mother liquor. Typical solubility curves are shown in Figure 1. Is the material a hydrated or anhydrous material? What is the solubility of the compound in water or any other solvents under consideration and how does this change with temperature and pH? Are there other compounds in the solution that coprecipitate or remain in solution, if so, how does their presence affect the solubility of the main component? What will be the influence of the impurities on the crystal habit and the growth and nucleation rate? What are the physical properties of the solution and the crystal? What vapor pressure of water exists over the saturated solution and crystals as a function of temperature? What is the heat of crystallization? What is the production rate and on what basis is this production rate computed? What materials of construction can be used in contact with the solutions at various temperatures? What utilities will be used at the crystallizer location and what are the costs associated with these utilities? Is the final product to be blended or mixed with other crystalline materials or solids? What size of product crystal is required and how can this material be separated from the mother liquor and dried? How can these solids or mixtures be handled and stored without undue breakage or caking?
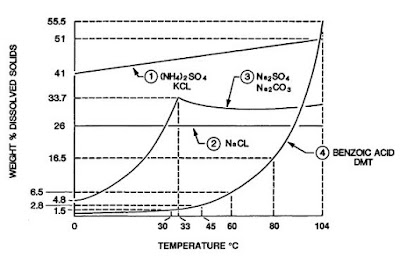 |
| Figure 1 Solubility versus temperature for various compounds. |
- Solubility:
In working with materials of very steep solubility, such as Glauber's salt or other hydrated salts, it is common to use cooling-type crystallizers since they permit very high overall yields and reduce the overall energy requirements of the separation process. With materials whose solubility curves have a normal but moderate slope, many choices are available including evaporative cooling, surface cooling, or constant temperature evaporation. With materials having flat or inverted solubility, it is necessary to use evaporative crystallization. This can be done by causing water to be evaporated from the solution at a wide variety of temperatures, depending on the economics, the crystal phase desired, and the materials of construction available. Evaporative crystallization is generally done at a constant temperature but can also be performed at relatively low temperatures in solar ponds. In general, cost considerations dictate that cooling crystallization is used in cases where the precipitated salts are highly hydrated or where the solubility curve is relatively steep. The reason for this is that to produce solids by cooling only requires removal of the heat of crystallization and the sensible heat of cooling the mother liquor. In general, these effects are quite small, typically 0.7cal/gm°C for cooling the solution and from 18-55 kcal/kg of product crystallized. To produce a pound of crystals by evaporation requires vaporization of water that is a function of the solubility, but typically might represent 1100 kcal/kg of product crystallized for a material whose solubility is 33%. Obviously, there are other factors that are highly specific to each case under consideration, but typically, surface cooling requires a somewhat higher investment than does evaporative cooling. In addition, the operating cycles of surface-cooled equipment tend to be shorter than those of evaporative crystallizers.
- Scale of Operation:
The scale of operation often has an overriding importance on the selection of the equipment because of the means used for heat transfer. For very small-scale crystallization work it is common to use radiation. The capacity of such equipment varies from a few liters up to several hundreds of liters per day (of solution cooled). For operation on scales up to several thousand liters per day, it is possible to use tanks with water-cooled coils and an agitator. For large-scale applications where the quantity of solution is thousands of liters per day, it is almost universal practice to use vacuum evaporation to remove the solvent; this is true whether the solution is cooled by adiabatic evaporation or in equipment where crystallization occurs because of isothermal evaporation.
- Batch Or Continuous Operation:
Another consideration is whether the crystallization should be carried out on a batch or continuous basis. The present tendency in most processing plants is to use continuous equipment wherever possible. Continuous equipment permits adjusting the operating conditions to a relatively fine degree to get the best results in terms of overall energy usage and product characteristics. It permits the use of a smaller operating labor force and results in a continuous utility demand that minimizes the size of boilers, cooling towers, and power generation facilities. It also minimizes the capital investment not only in the crystallizer but in the feed storage and product liquor storage facilities. Continuous separating devices for the product crystals have been developed to the point where they can operate reliably for long periods of time and the drying of crystalline products is also generally done on a continuous basis.
Batch handling of wet or semidry crystalline materials presents considerable difficulties as compared with the storing and handling of dried crystalline materials. On the other hand, a train of continuous processing equipment has economic application only on relatively large production rates from approximately 50 tons of product per day and upward.
Batch crystallization still has some range of application where production rates are very small, where very expensive materials are being handled and losses must be kept to an absolute minimum, or where a careful inventory of materials is required. Wall deposits in batch crystallizers are generally removed by each new charge of unsaturated feed. The batch operation also has a useful application where the cooling range is very wide, such as in handling material whose initial feed concentration corresponds to relatively high pressure and whose final mother liquor temperature corresponds to room temperatures or values significantly below it. In such systems, the use of batch crystallization avoids the shock introduced to the system by mixing high-temperature feed solutions with relatively low-temperature mother liquor in continuous equipment.
Another area of application is when the final liquor temperature is so low that it requires the use of extensive vapor compression equipment. In such systems, the use of batch equipment is frequently more economical than anything else, other than a relatively large number of multiple-stage series crystallizers.
- Multistage Crystallizers:
The use of multistage crystallization equipment, while not analogous to multistage evaporation equipment, nevertheless permits certain economics in operation. It is normally employed when a large flow at a high temperature and concentration is cooled to produce crystals and the mother liquors are returned to a dissolving or leaching station for reconcentration. In such systems, it is possible to use multistage cooling so that the mother liquor from the final crystallizer can be heated by crystallizers in the first one or several stages of the process. This heating can be done in either barometric heaters or in a tube and shell-type exchangers. The use of this type of system not only reduces the steam required to raise the leach solution to its operating temperature but also reduces the cooling water required on the last one or two stages of crystallization.
- Mechanical Vapor Recompression:
As the cost of energy has increased in recent years, attention is again being directed to mechanical vapor recompression, which by its nature, permits substitution of electrical energy for evaporation and crystallization rather than requiring heat energy (steam). In a single-stage evaporative crystallizer operating at approximately atmospheric pressure, the amount of heat energy necessary to remove a kilogram of water to produce the equivalent in crystal product is approximately 555 kcal. If the water evaporated is compressed by a mechanical compressor of high efficiency to a pressure where it can be condensed in the heat exchanger so as to supply the energy needed to sustain the process, then the equivalent power for this compression is about 6.3 kcal (Bennett 1978).
Although this technique is limited to those materials having relatively low boiling point elevation and to those cases wherein a significant amount of heat input is required to produce the evaporation required for a given crystallization step, it nevertheless offers an attractive technique for reducing the use of heat energy and substituting mechanical energy (or electrical energy) in those cases where there is a cost advantage in doing so. This technique finds many applications in the crystallization of sodium sulfate, NaCl, and sodium carbonate monohydrate.
- Reactive Crystallizers:
When a solid-phase crystalline material results from the reaction of two components, it is often advantageous to perform this reaction in a reactive crystallizer rather than in a separate reactor. This is commonly done, for example, in the case of ammonium sulfate where liquid or gaseous ammonia and concentrated sulfuric acid are reacted in the crystallizer to produce ammonium sulfate crystals, as shown in Figure 2. The reaction is carried on in a liquid suspension of growing crystals and the heat of reaction is removed by vaporizing water that is condensed and recycled to maintain the liquid balance. Other examples are the production of (NH4)2HP04 from NH3 and H3PO4 the oxidation of calcium sulfite by oxygen (air), and the neutralization of waste acid by lime.
The reactants can be mixed in the circulation piping of a forced-circulation-type crystallizer, or "Oslo" crystallizer or in the draft tube of a DTB-type crystallizer (Figure 2) where a large volume of slurry is mixed continuously with the reactants so as to minimize the driving force (supersaturation created by the reaction). Removal of heat is conveniently done by vaporizing water or other solvents as in a conventional evaporative-type crystallizer.
 |
Figure 2
Swenson reaction-type DTB crystallizer.
|



Here we come up with new machinery plant named Alaquainc, which specializes in designing and manufacturing a broad range of different crystallizer systems designs to fulfill diverse client requirements. To learn more about Alaqua Inc please visit on the website www.alaquainc.com
ReplyDeleteHi dear
ReplyDeleteThanks for your good explanation
Thank you for the helpful blog, "Crystallizer Selection And Design." I want you to know that your information is invaluable for aspiring candidates. Keep sharing valuable updates!
ReplyDeleteChandu Biology Classes